Change the trajectory of
osteoporosis for your patients.1
How TYMLOS rebuilds bone.1

TYMLOS is a PTHrP(1–34) analog that acts as an agonist at the PTH1 receptor. It activates the cAMP signaling pathway, which triggers the remodeling process in favor of bone formation.1,5-8
formation in open-label study
Watch the video to learn about abaloparatide’s mechanism of action.1
Video is intended to be viewed on desktop. If viewing on a mobile device, video should be played in full screen landscape mode.

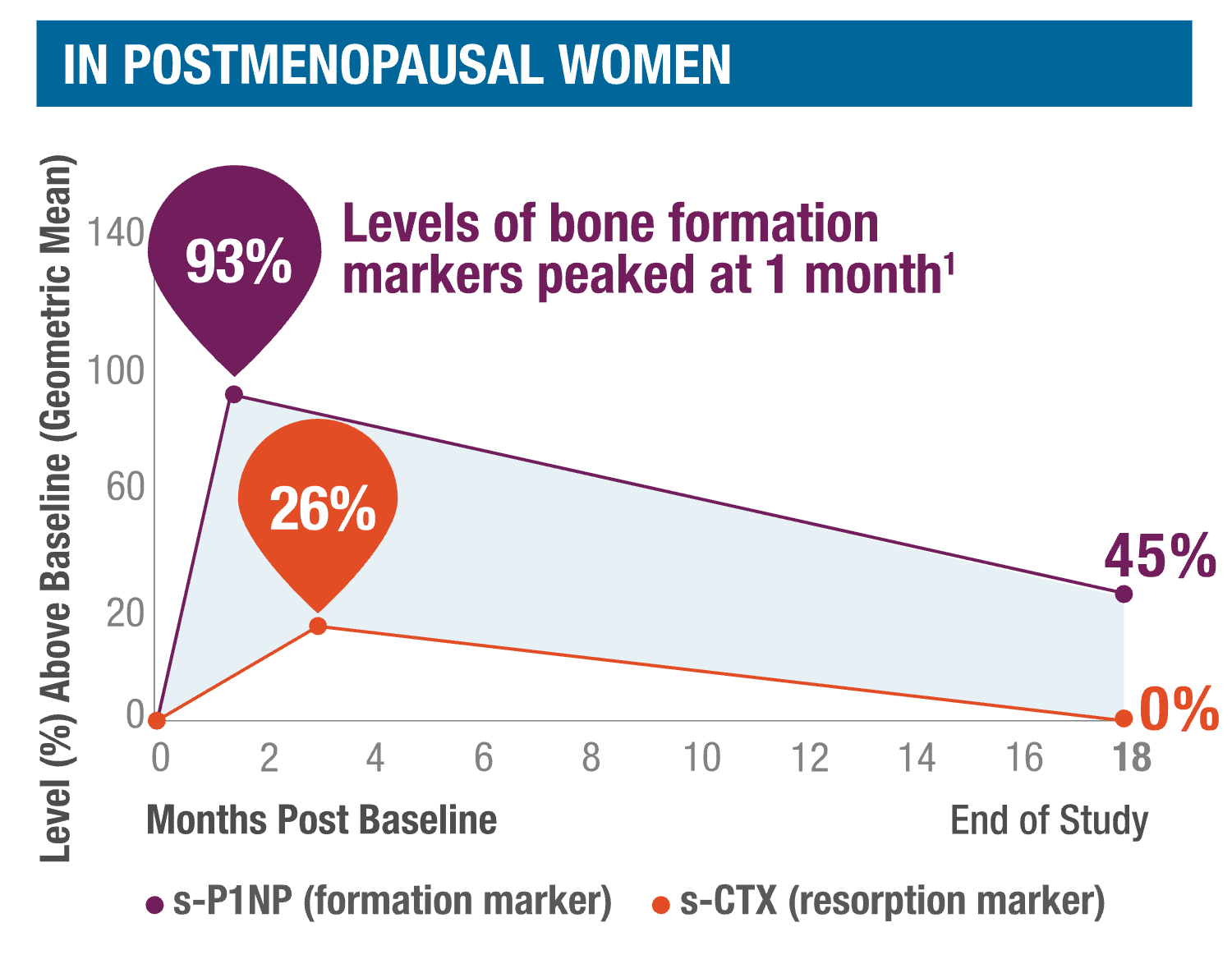
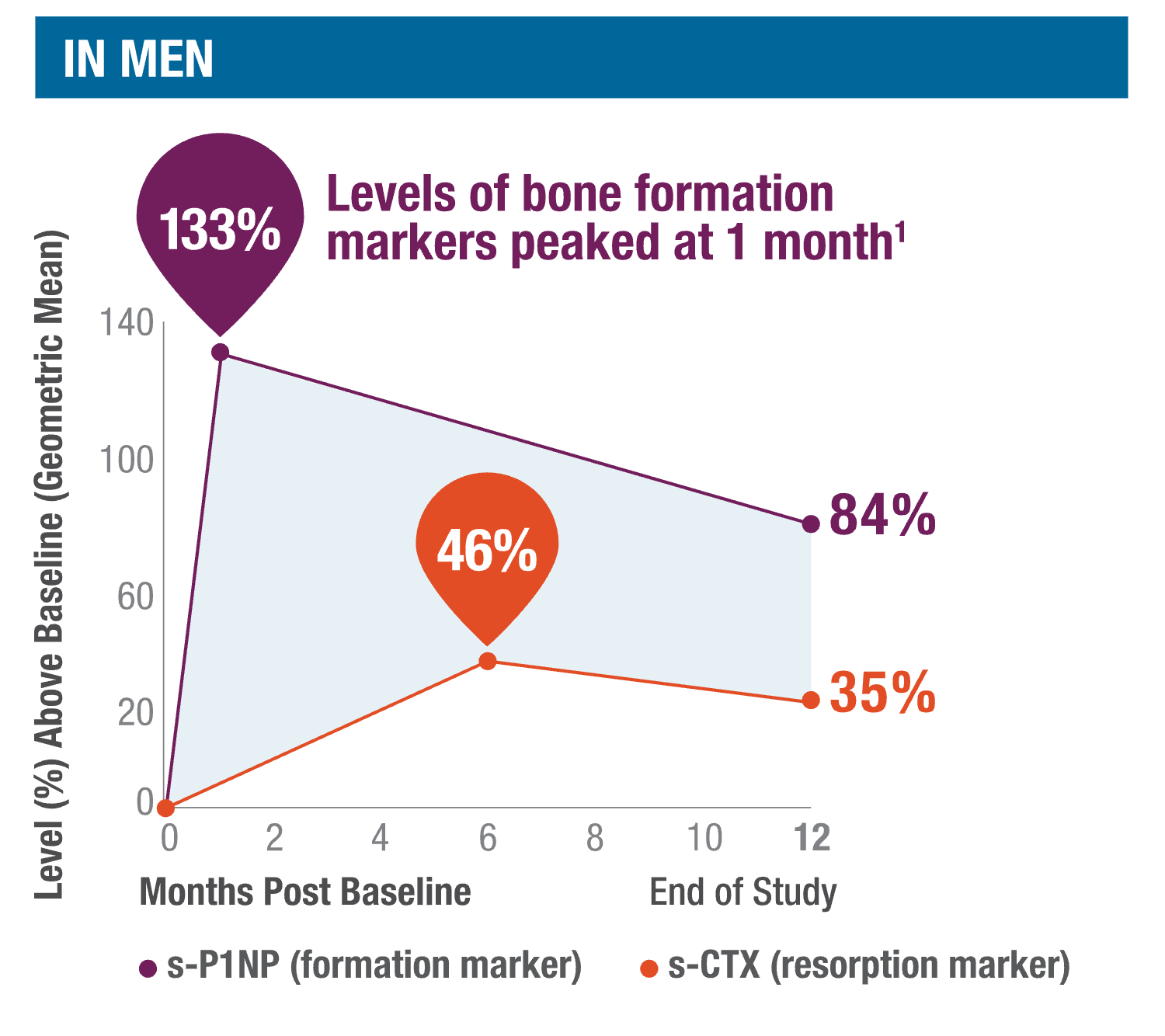
TYMLOS quickly stimulates, then sustains, bone formation.1
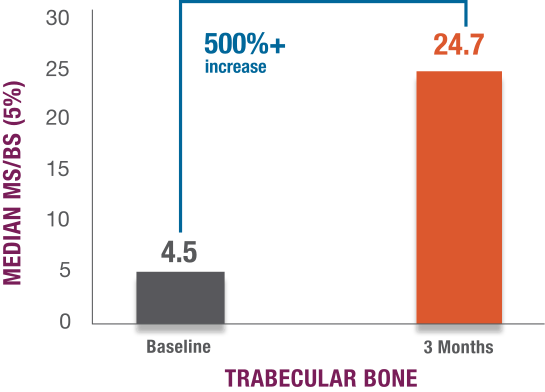
Mineralizing surface increase at 3 months*2
Mechanism of action representations are not meant to imply clinical efficacy. No new safety signals were seen. Adverse events were similar to those seen in the ACTIVE trial.1,2
*3 months is the amount of time it takes bones to form and mineralize in healthy adults.2-4
Open-label study evaluating the effects of TYMLOS on indices of bone formation.2
Study design: Open-label, single-arm study that evaluated the effects of TYMLOS on indices of bone formation using transiliac bone biopsies in postmenopausal women with osteoporosis. 23 biopsies were included in the safety population; 19 biopsies were evaluable. Subjects received double fluorochrome labels before treatment and before biopsy collection at 3 months.
Primary endpoint: Change in the mineralizing surface per unit of bone surface (MS/BS) in trabecular bone, from baseline to 3 months.
Secondary endpoints:
- MS/BS across cortical and periosteal surfaces2
- Remodeling- and modeling-based bone formation2
Inclusion criteria:
- Postmenopausal women with osteoporosis, aged 50-85 years2
- BMD: T-score ≤ –2.5 at the lumbar spine or hip (including femoral neck or total hip) OR BMD+Fracture History: T-score ≤ –2.0 at the lumbar spine or hip and a recent (>5 years) low-trauma vertebral, forearm, humerus, sacral, pelvic, femoral, or tibial fracture2
Exclusion criteria: Patients with unevaluable lumbar spine or hip BMD, a history of bone disorders, metabolic bone disease, malabsorption, cancer in the past 5 years, osteosarcoma at any time, prior radiotherapy (besides radioiodine), Cushing’s disease, or hypo- or hyperparathyroidism were excluded. Patients with a known hypersensitivity to TYMLOS, those who received prior treatment with PTH or PTHrP, denosumab, or IV bisphosphonates, took medications that interfere with bone metabolism within 6 months, or received treatment with oral bisphosphonates within 3 years were not eligible for this study.2
Study limitations2:
- Consider open-label study limitations when interpreting results. This open-label study was not blinded. These results are not meant to imply fracture efficacy
- Single-arm study with no placebo group comparison and included only caucasian female participants
- Since the study had no control arm, a quadruple labeling procedure was used for internal control, allowing for a baseline comparison of bone formation indices within the same sample
- Data generated from bone biopsies are variable, between subjects, and between biopsies taken from the same subject
- While significant changes in these parameters are unlikely after 3 months of treatment, this labeling procedure does limit the ability to evaluate the effect of treatment on bone microarchitectural endpoints
- Transiliac bone biopsies may not precisely reflect changes in bone at weight-bearing sites
BMD=bone mineral density; cAMP=cyclic adenosine monophosphate; IV=intravenous; PTH1=parathyroid hormone 1; PTHrP=parathyroid hormone-related peptide; s-CTX=serum carboxy-terminal cross-linked telopeptide of type 1 collagen; s-P1NP=serum procollagen type 1 N-terminal propeptide.
Dr. Michael Lewiecki discusses how men are often overlooked when identifying and treating osteoporosis.
View the presentation below (viewing in full screen is recommended).

Your patients could be at imminent risk for fracture
AACE/ACE guidelines define patients at very high risk for fracture based on†9:

- –3.0 or worse

- Recent (<1 year)
- Multiple fractures
- History of fracture while on osteoporosis treatment or drugs causing skeletal harm


Very high fracture probability:
- >30% major osteoporosis fracture
- >4.5% hip fracture
In addition, patients unable to use antiresorptive therapy or those that experience progressive bone loss while on antiresorptive therapy remain at elevated risk.
†AACE/ACE Guidelines are intended for postmenopausal women with osteoporosis.
AACE=American Association Of Clinical Endocrinologists, ACE=American College Of Endocrinology.
FRAX=Fracture Risk Assessment Tool, used to estimate the risk of fracture in the next 10 years.
FRAX is a registered trademark of the World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK.
AACE/ACE guidelines support the use of abaloparatide for appropriate patients

If patients have factors that put them at very high risk for fracture or are unable to take an antiresorptive

If patients on antiresorptive therapy experience progressive bone loss, a fracture, or treatment intolerance
Dr. David Dempster discusses TYMLOS as a treatment option for both men and postmenopausal women that rebuilds bone.
View the presentation below (viewing in full screen is recommended).

TYMLOS is self-administered and requires no refrigeration after first use.1
Store for up to 30 days without refrigeration at a temperature between 68°F and 77°F (20°C and 25°C).1

See how TYMLOS performed in clinical trials
Resources and support for you and your patients
Let us assist you with injection training and more.
Need support or samples?
Let us know if you want to request samples or a visit from a TYMLOS representative or field reimbursement manager.



